1. Test method summary
The sample is washed with ethanol to remove the soluble salt, then dried and burned at high temperature. The residue is sodium oxide, dissolved in water to form sodium hydroxide, add over-dose sulfuric acid standard titration solution, using sodium hydroxide standard titration solution titrate excess sulfuric acid, by calculating the average value of the carboxylmethyl group in each anhydrous glucose units . The average value of the carboxylmethyl group is the degree of substitution.
2. Reagents and materials
1) Anhydrous ethanol
2) Ethanol solution: take 90mL of the ethanol, diluted with water to 100mL.
3) Sulfuric acid standard titration solution :
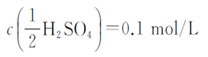
4) Sodium hydroxide standard titration solution:
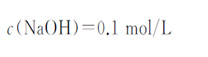
5) Methyl red indicator solution: 1g/L.
3. Test equipment
1)Glass sand crucible: Filter plate aperture 15μm~40μm
2)Porcelain crucible: 20mL-25mL.
4. Steps
Approximately weigh 1.5 g sample and place in a glass sand crucible and washed several times with an ethanol solution which preheated to 50 ° C to 70 ° C (each time the glass sand crucible is filled with ethanol solution) until add one drop of potassium chromate solution and one drop the filtrate of silver nitrate solution was showed brick red color, it means the washing was done, otherwise should keep washing, generally wash 5 times.The last time use anhydrous ethanol washing, put the washed sample into the glass sand crucible with 120 ℃ ± 2 ℃ drying 2h (when it has passed 1h , the glass sand crucible sample should be knocked to be loose ).
Combined with the lid and moved into the dryer and cooled to room temperature. Weigh about 1g of sample, accurate to 0.0002g, placed in the porcelain crucible, on the electric furnace carbonized to no smoke, put into the 300 ℃ high temperature furnace, heating up to 700 ℃ ± 25 ℃, keep warm for 15min, turn off the power, cool to 200 ℃ below, put into the 250mL beaker, add 100mL water and 50mL ± 0.05mL sulfuric acid standard titration solution, heating the beaker on the electric furnace , slowly boiling 10min, add 2 drops ~ 3 drops of methyl red indicator solution, cooling, with sodium hydroxide Standard titration solution titrated to red color just faded.
5. Testing result calculating:
Degree of substitution : xD.S ,according to A.2 and A.3 calculating.
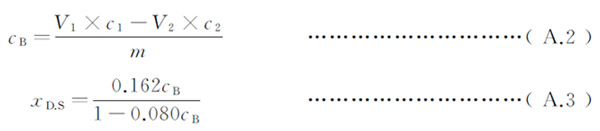
CB – The number of moles of carboxymethyl in the sample, unit is millimoles per gram;
V1 – Sulfuric acid Standard titration solution volume, unit is milliliters;
C1 – The actual concentration of sulfuric acid standard titration solution, unit is moles per liter (mol / L);
V2 – Sodium hydroxide standard titration solution volume, unit is milliliters (mL);
C2 – The actual concentration of sodium hydroxide standard titration solution, unit is moles per liter (mol / L);
m -The weight of the sample, unit is grams (g);
0.162-The molar mass of a glucose unit weight in cellulose, unit is grams per millimolar (g / mmol);
0.080 – the carboxymethyl sodium group in millimolar mass, unit is grams per millimolar (g / mmol);
The result of the calculation indicates 2 digits after the decimal point. The arithmetic mean of the results of two parallel measurements was taken as the determinations result.
The absolute difference is not beyond than 0.02.


SINOCMC Team
