Summary of testing method
Dissolve the sample in water, titrate with silver nitrate titrant, and use the potentiometer to indicate the end point. Add hydrogen peroxide to the solution to degrade the viscosity.
Reagents and materials
nitric acid, hydrogen peroxide, and silver nitrate titrant: c(AgNO3)=0.1mol/L
Test instruments
Potentiometric titrator: with silver electrode and saturated calomel electrode (double salt bridge).
Micro burette: 10mL.
Steps
Weigh 2g samples dried at 105 ℃±2 ℃ for 2h, accurate to 0.0002g. Put it in a 250mL beaker, add 100mL water and 5mL hydrogen peroxide. Heat the beaker in a steam bath, stirring intermittently to obtain a non-sticky solution. If the solution viscosity is not completely degraded after 20 minutes, add another 5mL hydrogen peroxide and heat until the degradation is complete. Cool the beaker, add 100mL water and 10mL nitric acid, place it on the magnetic agitator of the potentiometric titrator, and add silver nitrate titrant to the potentiometric end point with a micro burette.
Testing result calculating
The mass fraction w2 of chloride content (NaCl) is calculated according to Equation (A.5):
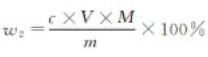
c — silver nitrate standard solution concentration, in moles per liter (mol/L);
V — Volume of silver nitrate standard titration solution, expressed in milliliters (mL);
M — Millimole mass of sodium chloride, expressed in grams per millimole (g/mmol)(M =0.05845);
m — The mass of the sample, expressed in grams (g).
The result is expressed to one decimal. The average of the results of two parallel measurements is taken as the measurement results, and the absolute difference between the results of two parallel measurements is no more than 0.06%.
If you have any question about the determination of chloride in food grade Sodium CMC, please contact SINOCMC freely.
SINOCMC TEAM
