Sodium Carboxymethyl Cellulose(SCMC) is one kind of cellulose derivatives which can be widely used in food, detergent, paper making, ceramic and painting industries etc. We would like to introduce Sodium CMC formula and its characteristics.
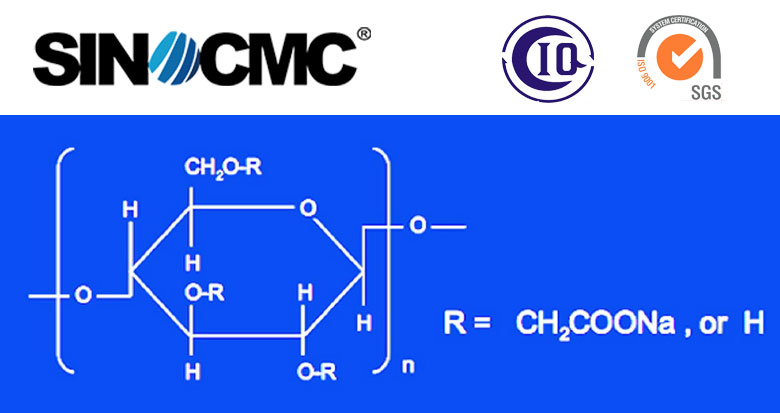
Sodium CMC is anionic cellulose which is made from refined cotton(or wooden pulp), liquid Sodium Hydroxide and Monochloroacetic Acid. The formula [C6H7O2(OH)2OCH2COONa]n and it is shaped by carboxymethyl groups (-CH2-COOH) connected with some of the hydroxyl groups of the glucopyranose monomers and Sodium that make up the cellulose backbone. So it is named Sodium salt and belongs to organic compound.
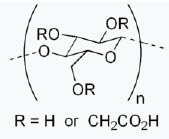
Carboxymethyl Cellulose structure formula
There are total three chemical reaction during the whole production:
(1). C6H7O2(OH)2OCH2COOH+NaOH C6H7O2(OH)2OCH2COONa+H2O
(Alkalization Reaction)
(2). ClCH2COOH+NAOH ClCH2OONa+H2O
(3). C6H7O2(OH)2OCH2COO-Na +ClCH2OO- C6H7O2(OH)2OCH2COO-Na
(Etherification Reaction)
Definitely, some byproducts will be produced together, such as: NaCl, Sodium Glycolate and Hydroxyacetic Acid Salt etc. Sodium CMC can’t be dissolved in ethanol and other byproducts can be dissolved in ethanol, so we can wash out the byproducts and increase CMC purity via first, twice and three times washing, and purity can reach 85%, 95%, 99.5%.

Various types of Sodium CMC(granular CMC, powder CMC, ultra high viscosity CMC, low viscosity CMC) can be provided by us. If you have any question about our products, please contact SINOCMC freely.
SINOCMC Team
